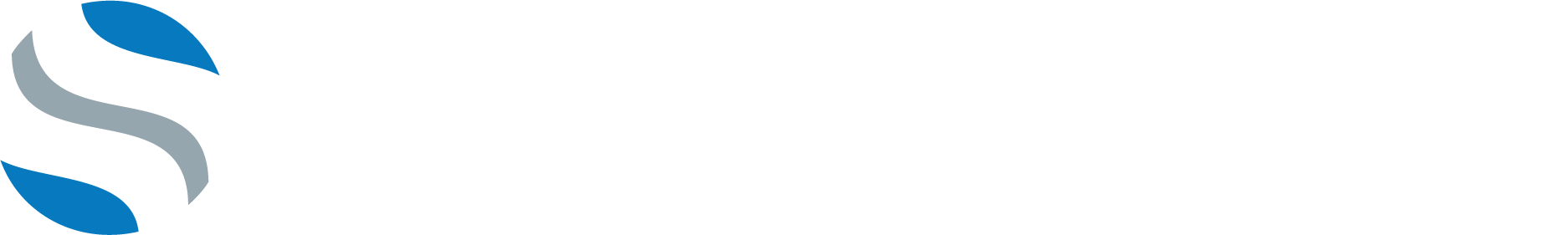• Zero-profile implant design
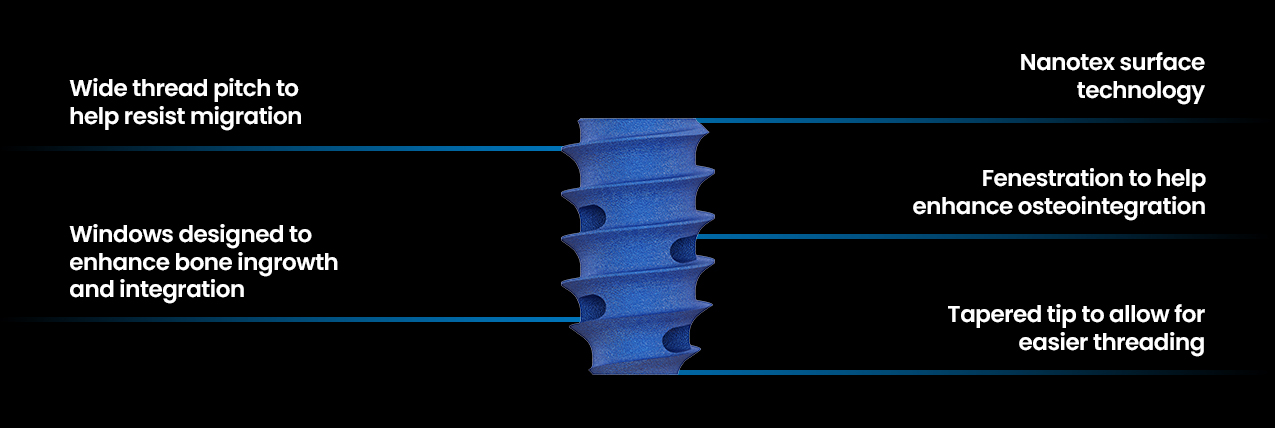
ION-C™ Key Features
Stabilization Without Distraction
ION-C™ is a posterior cervical facet fixation system designed for controlled, non-impact placement and precise stabilization across one or two levels from C3–C7. When ION-C™ is implanted the joint is kept in a neutral position, reducing the risk of joint expansion while it’s engagement features are designed to resist implant expulsion.

Multiple Configurations Available
ION-C™ Instruments are offered in various configurations, including single-use (provided sterile), and reusable (provided non-sterile). The Ion-C Instruments may be used to rasp or decorticate bone from the facets and/or transverse processes and for the delivery of bone graft.
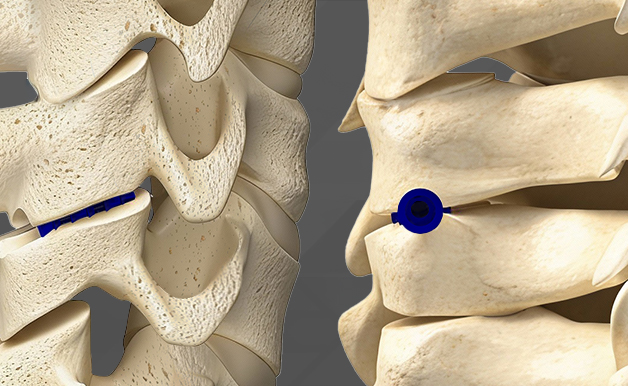
Minimally Invasive Surgery
Typically a small incision is utilized to minimize tissue disruption and scarring. This approach is designed with safety in mind, offering patients a less invasive alternative to traditional spine surgery. ION-C™ offers controlled implant depth by allowing in situ depth adjustment.
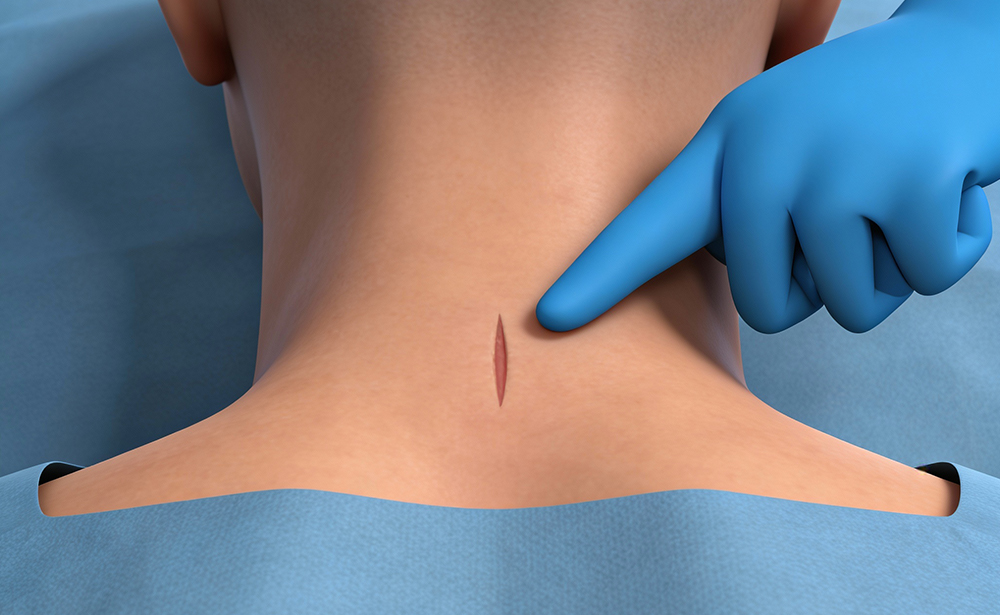
Osseointegration Promotion
Hydrophilic surface created by Nanotex®1 technology takes advantage of ionic bonds to attract and hold BMA, and other fluids necessary for osseointegration. The ION-C™ offers a zero profile implant design which includes fenestrations and open barrels allow for bone graft integration and fusion.
ION-C™ Design Highlights
Imaging Results
1-Level Cervical Pseudoarthrosis
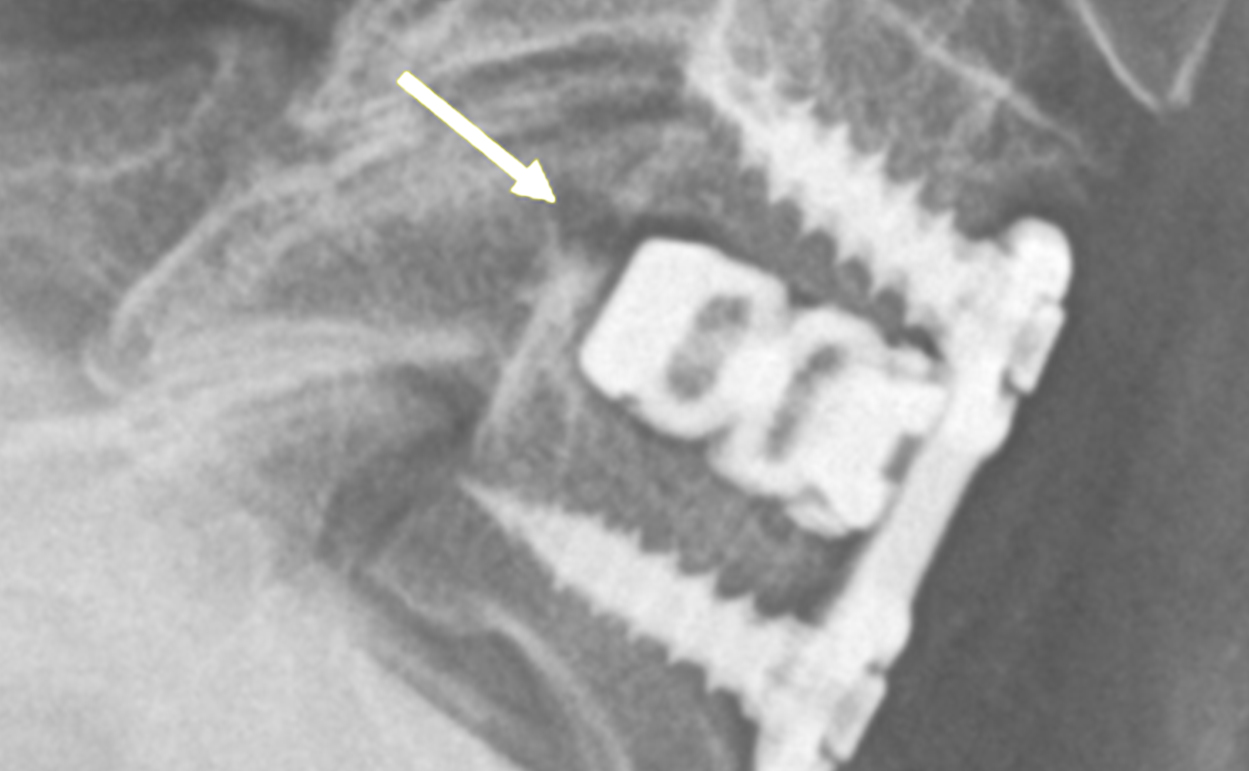
Arrow depicts baseline xray psuedoarthrosis posterior of cage.
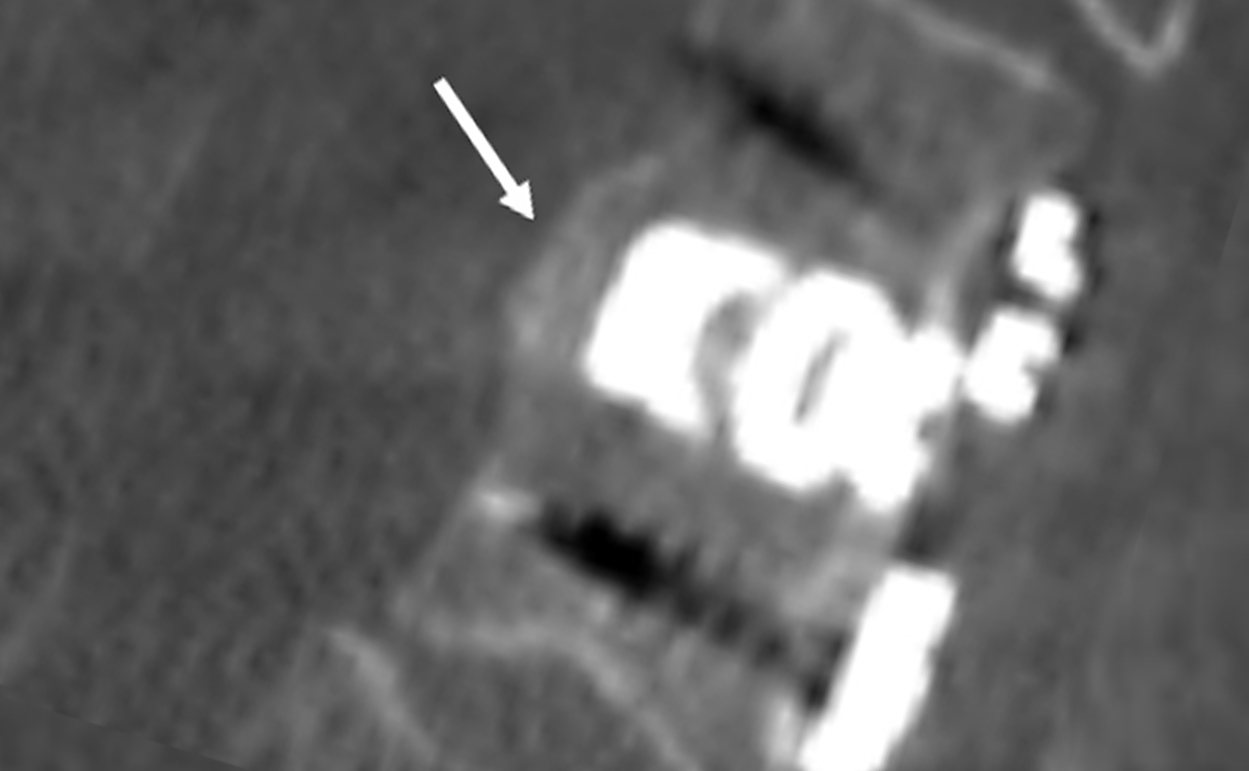
CT scan imaging 16 months post op demonstrates osseous continuity posterior of cage.
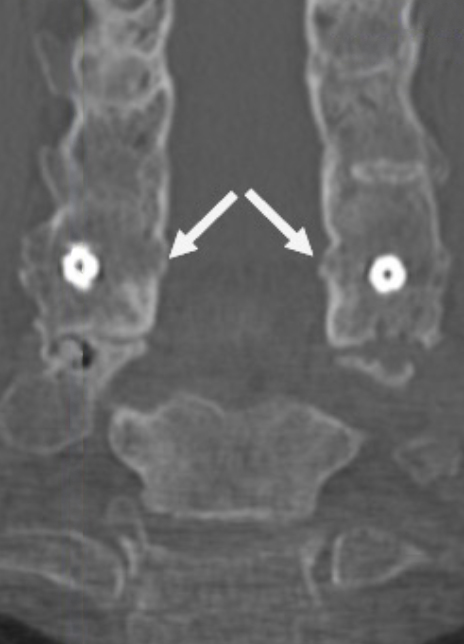
Coronal CT image showing circumferential bone bridging around the ION-C implants bilaterally.
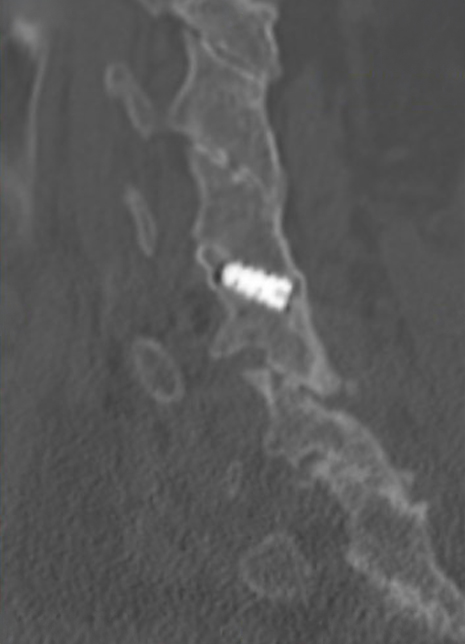
Sagittal CT image showing left anterior/posterior bone bridging.
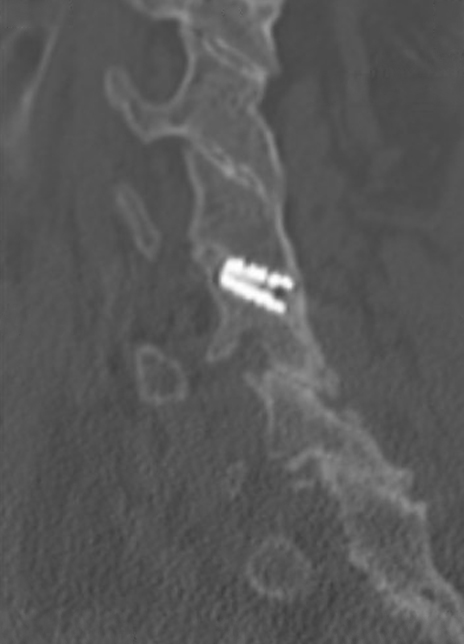
Sagittal CT image showing right anterior/posterior bone bridging.
2-Level Cervical Pseudoarthrosis
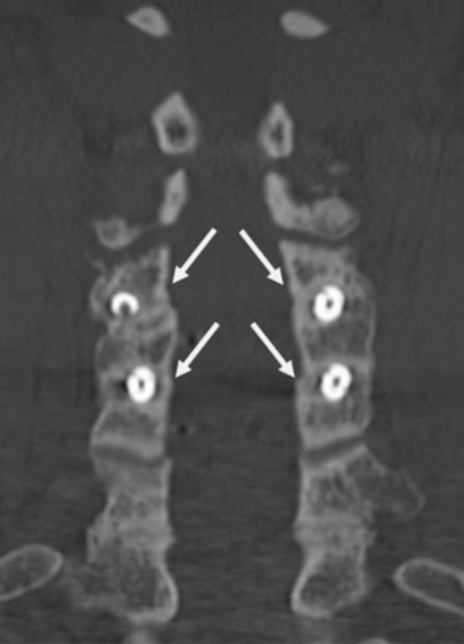
Coronal CT image showing circumferential bone bridging around the ION-C implants bilaterally.
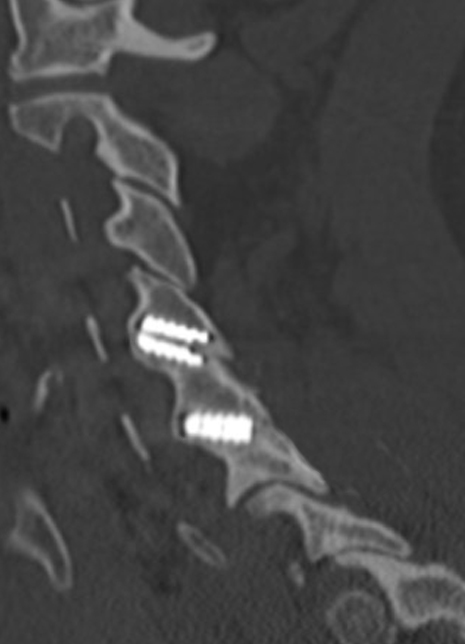
Sagittal CT image showing anterior bone bridging.
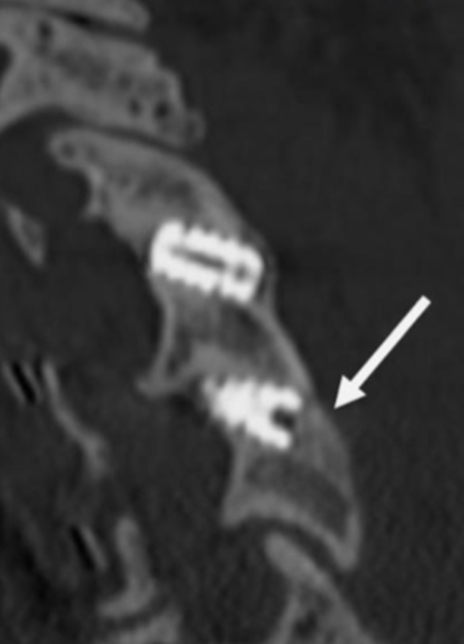
Sagittal CT image showing posterior bone bridging.
Histology Review
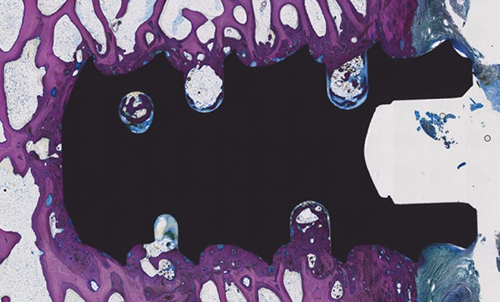
Ovine spine study at three (3) months revealed
mature bone formation to Nanotex® surface with no
delamination
This information is based on an ovine study of the Ion-C device, and may not be representative of clinical performance1
Blood Wicking
 The hydrophilic surface created by Nanotex® technology takes advantage of ionic bonds to attract and hold blood, BMA, and other fluids that may contain key growth factors necessary for osseointegration
The hydrophilic surface created by Nanotex® technology takes advantage of ionic bonds to attract and hold blood, BMA, and other fluids that may contain key growth factors necessary for osseointegration
The above claim is based on an ovine study and may not be representative of clinical performance1
INDICATIONS FOR USE/INTENDED USE
ION-C™ is intended to be placed bilaterally through a posterior surgical approach and spans the facet joint interspace. The ION-C™ is intended for temporary stabilization as an adjunct to posterior cervical fusion in skeletally mature patients. The ION-C™ is indicated for patients requiring a revision for an anterior pseudoarthrosis at one or two contiguous levels, from C3 to C7. The ION-C™ is only intended to be used in combination with an FDA-cleared anterior cervical plate and intervertebral body fusion device implanted at the same level(s). The ION-C™ is intended for use with autogenous and/or allogenic bone graft.